Where Is Collagen Found In The Skin

Tropocollagen molecule: three left-handed procollagens (ruby, green, blue) join to form a correct-handed triple helical tropocollagen.
Collagen () is the chief structural protein in the extracellular matrix plant in the body's various connective tissues. As the master component of connective tissue, information technology is the about arable protein in mammals,[1] making up from 25% to 35% of the whole-body protein content. Collagen consists of amino acids spring together to form a triple helix of elongated fibril[ii] known as a collagen helix. It is more often than not constitute in connective tissue such as cartilage, bones, tendons, ligaments, and skin.
Depending upon the caste of mineralization, collagen tissues may exist rigid (bone) or compliant (tendon) or have a slope from rigid to compliant (cartilage). Collagen is also abundant in corneas, blood vessels, the gut, intervertebral discs, and the dentin in teeth.[3] In muscle tissue, it serves as a major component of the endomysium. Collagen constitutes 1 to two per centum of musculus tissue and accounts for half dozen% of the weight of strong, tendinous muscles.[iv] The fibroblast is the most common cell that creates collagen. Gelatin, which is used in food and industry, is collagen that has been irreversibly hydrolyzed.[5] Collagen has many medical uses in treating complications of the bones and skin.
Etymology [edit]
The name collagen comes from the Greek κόλλα (kólla), pregnant "mucilage", and suffix -γέν, -gen, cogent "producing".[6] [vii]
Homo types [edit]
Over 90% of the collagen in the homo trunk is type I collagen.[8] However, as of 2011, 28 types of human collagen take been identified, described, and divided into several groups according to the construction they class.[9] All of the types incorporate at least ane triple helix.[ix] The number of types shows collagen'south diverse functionality.[10]
- Fibrillar (Type I, II, III, Five, Xi)
- Non-fibrillar
- FACIT (Fibril Associated Collagens with Interrupted Triple Helices) (Type Ix, XII, Fourteen, XIX, XXI)
- Brusque chain (Blazon Eight, X)
- Basement membrane (Type IV)
- Multiplexin (Multiple Triple Helix domains with Interruptions) (Type 15, Eighteen)
- MACIT (Membrane Associated Collagens with Interrupted Triple Helices) (Type XIII, XVII)
- Microfibril forming (Type Vi)
- Anchoring fibrils (Type VII)
The 5 virtually common types are:[11]
- Type I: skin, tendon, vasculature, organs, os (primary component of the organic role of bone)
- Type 2: cartilage (main collagenous component of cartilage)
- Type Three: reticulate (main component of reticular fibers), commonly constitute alongside type I
- Type IV: forms basal lamina, the epithelium-secreted layer of the basement membrane
- Type Five: cell surfaces, hair, and placenta
Medical uses [edit]
Cardiac applications [edit]
The collagenous cardiac skeleton which includes the 4 eye valve rings, is histologically, elastically and uniquely bound to cardiac muscle. The cardiac skeleton too includes the separating septa of the heart chambers – the interventricular septum and the atrioventricular septum. Collagen contribution to the measure of cardiac performance summarily represents a continuous torsional forcefulness opposed to the fluid mechanics of claret pressure emitted from the heart. The collagenous construction that divides the upper chambers of the eye from the lower chambers is an impermeable membrane that excludes both claret and electrical impulses through typical physiological means. With support from collagen, atrial fibrillation never deteriorates to ventricular fibrillation. Collagen is layered in variable densities with smooth muscle mass. The mass, distribution, age and density of collagen all contribute to the compliance required to motility blood back and along. Individual cardiac valvular leaflets are folded into shape by specialized collagen under variable pressure. Gradual calcium deposition inside collagen occurs equally a natural part of aging. Calcified points within collagen matrices show dissimilarity in a moving display of blood and muscle, enabling methods of cardiac imaging technology to arrive at ratios essentially stating blood in (cardiac input) and blood out (cardiac output). Pathology of the collagen underpinning of the heart is understood within the category of connective tissue affliction.[ citation needed ]
Corrective surgery [edit]
Collagen has been widely used in cosmetic surgery, as a healing help for burn patients for reconstruction of os and a broad variety of dental, orthopedic, and surgical purposes. Both human and bovine collagen is widely used every bit dermal fillers for treatment of wrinkles and peel aging.[12] Some points of interest are:
- When used cosmetically, there is a chance of allergic reactions causing prolonged redness; withal, this can exist well-nigh eliminated by simple and inconspicuous patch testing prior to cosmetic utilise.[xiii]
- Nigh medical collagen is derived from immature beef cattle (bovine) from certified BSE-free animals. Almost manufacturers use donor animals from either "closed herds", or from countries which accept never had a reported example of BSE such as Australia, Brazil, and New Zealand.[13]
Os grafts [edit]
Equally the skeleton forms the structure of the body, it is vital that information technology maintains its force, even after breaks and injuries. Collagen is used in bone grafting as it has a triple helical structure, making it a very strong molecule. It is platonic for use in basic, as information technology does not compromise the structural integrity of the skeleton. The triple helical structure of collagen prevents it from being broken downwards by enzymes, it enables adhesiveness of cells and it is of import for the proper assembly of the extracellular matrix.[14]
Tissue regeneration [edit]
Collagen scaffolds are used in tissue regeneration, whether in sponges,[15] thin sheets,[16] gels,[17] or fibers.[18] Collagen has favorable properties for tissue regeneration, such equally pore structure, permeability, hydrophilicity, and stability in vivo. Collagen scaffolds likewise back up deposition of cells, such as osteoblasts and fibroblasts, and once inserted, facilitate growth to proceed normally.[19]
Reconstructive surgical uses [edit]
Collagens are widely employed in the construction of artificial skin substitutes used in the direction of severe burns and wounds.[twenty] [21] These collagens may be derived from bovine, equine, porcine, or even human sources; and are sometimes used in combination with silicones, glycosaminoglycans, fibroblasts, growth factors and other substances.[22]
Wound healing [edit]
Collagen is ane of the body's central natural resources and a component of pare tissue that can benefit all stages of wound healing.[23] When collagen is made available to the wound bed, closure tin can occur. Wound deterioration, followed sometimes by procedures such every bit amputation, tin thus be avoided.
Collagen is a natural product and is thus used as a natural wound dressing and has properties that artificial wound dressings do not have. It is resistant confronting bacteria, which is of vital importance in a wound dressing. It helps to go along the wound sterile, considering of its natural ability to fight infection. When collagen is used equally a burn dressing, healthy granulation tissue is able to grade very speedily over the burn, helping information technology to heal rapidly.[24]
Throughout the four phases of wound healing, collagen performs the following functions in wound healing:
- Guiding role: Collagen fibers serve to guide fibroblasts. Fibroblasts drift along a connective tissue matrix.
- Chemotactic backdrop: The large surface surface area available on collagen fibers tin attract fibrogenic cells which help in healing.
- Nucleation: Collagen, in the presence of certain neutral salt molecules can act equally a nucleating agent causing germination of fibrillar structures. A collagen wound dressing might serve as a guide for orienting new collagen deposition and capillary growth.
- Hemostatic properties: Blood platelets interact with the collagen to make a hemostatic plug.
Basic research [edit]
Collagen is used in laboratory studies for jail cell culture, studying cell behavior and cellular interactions with the extracellular environs.[25] Collagen is as well widely used as a bioink for 3D bioprinting and biofabrication of 3D tissue models.
Biological science [edit]
The collagen poly peptide is composed of a triple helix, which generally consists of ii identical chains (α1) and an boosted chain that differs slightly in its chemic composition (α2).[26] The amino acid composition of collagen is atypical for proteins, peculiarly with respect to its high hydroxyproline content. The almost mutual motifs in the amino acid sequence of collagen are glycine-proline-X and glycine-X-hydroxyproline, where X is any amino acid other than glycine, proline or hydroxyproline. The average amino acid limerick for fish and mammal pare is given.[27]
| Amino acrid | Abundance in mammal skin (residues/yard) | Affluence in fish pare (residues/1000) |
|---|---|---|
| Glycine | 329 | 339 |
| Proline | 126 | 108 |
| Alanine | 109 | 114 |
| Hydroxyproline | 95 | 67 |
| Glutamic acid | 74 | 76 |
| Arginine | 49 | 52 |
| Aspartic acid | 47 | 47 |
| Serine | 36 | 46 |
| Lysine | 29 | 26 |
| Leucine | 24 | 23 |
| Valine | 22 | 21 |
| Threonine | 19 | 26 |
| Phenylalanine | 13 | 14 |
| Isoleucine | 11 | 11 |
| Hydroxylysine | 6 | 8 |
| Methionine | 6 | 13 |
| Histidine | 5 | 7 |
| Tyrosine | 3 | three |
| Cysteine | 1 | ane |
| Tryptophan | 0 | 0 |
Synthesis [edit]
Get-go, a 3-dimensional stranded structure is assembled, with the amino acids glycine and proline as its principal components. This is non yet collagen simply its precursor, procollagen. Procollagen is and so modified by the addition of hydroxyl groups to the amino acids proline and lysine. This stride is of import for later glycosylation and the germination of the triple helix construction of collagen. Because the hydroxylase enzymes that perform these reactions require vitamin C as a cofactor, a long-term deficiency in this vitamin results in impaired collagen synthesis and scurvy.[28] These hydroxylation reactions are catalyzed past two different enzymes: prolyl-4-hydroxylase[29] and lysyl-hydroxylase. The reaction consumes one ascorbate molecule per hydroxylation. [30] The synthesis of collagen occurs inside and exterior of the jail cell. The germination of collagen which results in fibrillary collagen (well-nigh mutual form) is discussed hither. Meshwork collagen, which is often involved in the formation of filtration systems, is the other course of collagen. All types of collagens are triple helices, and the differences lie in the brand-up of the alpha peptides created in stride 2.
- Transcription of mRNA: Almost 44 genes are associated with collagen formation, each coding for a specific mRNA sequence, and typically accept the "COL" prefix. The showtime of collagen synthesis begins with turning on genes which are associated with the formation of a item alpha peptide (typically alpha 1, ii or 3).
- Pre-pro-peptide formation: In one case the last mRNA exits from the cell nucleus and enters into the cytoplasm, it links with the ribosomal subunits and the procedure of translation occurs. The early/first part of the new peptide is known as the signal sequence. The signal sequence on the Northward-terminal of the peptide is recognized by a bespeak recognition particle on the endoplasmic reticulum, which will be responsible for directing the pre-pro-peptide into the endoplasmic reticulum. Therefore, in one case the synthesis of new peptide is finished, it goes straight into the endoplasmic reticulum for post-translational processing. It is now known every bit preprocollagen.
- Pre-pro-peptide to pro-collagen: Iii modifications of the pre-pro-peptide occur leading to the formation of the alpha peptide:
- The betoken peptide on the Due north-terminal is removed, and the molecule is at present known every bit propeptide (non procollagen).
- Hydroxylation of lysines and prolines on propeptide by the enzymes 'prolyl hydroxylase' and 'lysyl hydroxylase' (to produce hydroxyproline and hydroxylysine) occurs to aid cross-linking of the alpha peptides. This enzymatic step requires vitamin C as a cofactor. In scurvy, the lack of hydroxylation of prolines and lysines causes a looser triple helix (which is formed past three blastoff peptides).
- Glycosylation occurs by adding either glucose or galactose monomers onto the hydroxyl groups that were placed onto lysines, simply non on prolines.
- In one case these modifications have taken identify, three of the hydroxylated and glycosylated propeptides twist into a triple helix forming procollagen. Procollagen yet has unwound ends, which will be afterwards trimmed. At this signal, the procollagen is packaged into a transfer vesicle destined for the Golgi apparatus.
- Golgi appliance modification: In the Golgi apparatus, the procollagen goes through one last post-translational modification before existence secreted out of the cell. In this step, oligosaccharides (non monosaccharides as in stride 3) are added, and and so the procollagen is packaged into a secretory vesicle destined for the extracellular space.
- Formation of tropocollagen: Once outside the cell, membrane bound enzymes known as collagen peptidases, remove the "loose ends" of the procollagen molecule. What is left is known as tropocollagen. Defects in this stride produce ane of the many collagenopathies known as Ehlers-Danlos syndrome. This step is absent when synthesizing type Iii, a type of fibrillar collagen.
- Formation of the collagen fibril: lysyl oxidase, an extracellular copper-dependent enzyme, produces the final step in the collagen synthesis pathway. This enzyme acts on lysines and hydroxylysines producing aldehyde groups, which volition eventually undergo covalent bonding between tropocollagen molecules. This polymer of tropocollagen is known as a collagen fibril.

Amino acids [edit]
Collagen has an unusual amino acid composition and sequence:
- Glycine is found at almost every 3rd residue.
- Proline makes up about 17% of collagen.
- Collagen contains ii uncommon derivative amino acids not directly inserted during translation. These amino acids are found at specific locations relative to glycine and are modified post-translationally by different enzymes, both of which require vitamin C as a cofactor.
- Hydroxyproline derived from proline
- Hydroxylysine derived from lysine – depending on the type of collagen, varying numbers of hydroxylysines are glycosylated (mostly having disaccharides attached).
Cortisol stimulates degradation of (peel) collagen into amino acids.[31]
Collagen I formation [edit]
Well-nigh collagen forms in a similar style, only the following procedure is typical for type I:
- Inside the jail cell
- Two types of alpha chains – alpha-1 and alpha ii, are formed during translation on ribosomes along the rough endoplasmic reticulum (RER). These peptide bondage known equally preprocollagen, have registration peptides on each cease and a signal peptide.[32]
- Polypeptide chains are released into the lumen of the RER.
- Signal peptides are cleaved inside the RER and the chains are now known as pro-alpha chains.
- Hydroxylation of lysine and proline amino acids occurs inside the lumen. This procedure is dependent on and consumes ascorbic acid (vitamin C) as a cofactor.
- Glycosylation of specific hydroxylysine residues occurs.
- Triple alpha helical construction is formed inside the endoplasmic reticulum from ii alpha-i chains and one alpha-two chain.
- Procollagen is shipped to the Golgi apparatus, where information technology is packaged and secreted into extracellular space by exocytosis.
- Outside the cell
- Registration peptides are broken and tropocollagen is formed by procollagen peptidase.
- Multiple tropocollagen molecules grade collagen fibrils, via covalent cross-linking (aldol reaction) by lysyl oxidase which links hydroxylysine and lysine residues. Multiple collagen fibrils course into collagen fibers.
- Collagen may be attached to jail cell membranes via several types of poly peptide, including fibronectin, laminin, fibulin and integrin.
Synthetic pathogenesis [edit]
Vitamin C deficiency causes scurvy, a serious and painful disease in which lacking collagen prevents the formation of strong connective tissue. Gums deteriorate and bleed, with loss of teeth; peel discolors, and wounds practise not heal. Prior to the 18th century, this condition was notorious among long-duration military, specially naval, expeditions during which participants were deprived of foods containing vitamin C.
An autoimmune disease such equally lupus erythematosus or rheumatoid arthritis[33] may attack healthy collagen fibers.
Many leaner and viruses secrete virulence factors, such as the enzyme collagenase, which destroys collagen or interferes with its production.
Molecular structure [edit]
A single collagen molecule, tropocollagen, is used to brand up larger collagen aggregates, such every bit fibrils. Information technology is approximately 300 nm long and one.five nm in diameter, and information technology is made upward of three polypeptide strands (called alpha peptides, come across step ii), each of which has the conformation of a left-handed helix – this should non exist confused with the correct-handed blastoff helix. These 3 left-handed helices are twisted together into a right-handed triple helix or "super helix", a cooperative 4th construction stabilized by many hydrogen bonds. With blazon I collagen and perhaps all fibrillar collagens, if not all collagens, each triple-helix associates into a correct-handed super-super-whorl referred to equally the collagen microfibril. Each microfibril is interdigitated with its neighboring microfibrils to a degree that might suggest they are individually unstable, although within collagen fibrils, they are then well ordered equally to be crystalline.
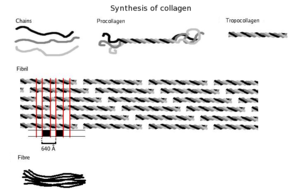
Three polypeptides curlicue to form tropocollagen. Many tropocollagens then demark together to form a fibril, and many of these then form a fibre.
A distinctive feature of collagen is the regular arrangement of amino acids in each of the three bondage of these collagen subunits. The sequence frequently follows the design Gly-Pro-X or Gly-10-Hyp, where X may exist whatsoever of diverse other amino acid residues.[27] Proline or hydroxyproline constitute about 1/6 of the total sequence. With glycine bookkeeping for the ane/3 of the sequence, this means approximately half of the collagen sequence is not glycine, proline or hydroxyproline, a fact often missed due to the lark of the unusual GXiXii graphic symbol of collagen alpha-peptides. The loftier glycine content of collagen is important with respect to stabilization of the collagen helix as this allows the very close association of the collagen fibers within the molecule, facilitating hydrogen bonding and the germination of intermolecular cross-links.[27] This kind of regular repetition and high glycine content is found in only a few other fibrous proteins, such as silk fibroin.
Collagen is not merely a structural protein. Due to its key role in the determination of cell phenotype, cell adhesion, tissue regulation, and infrastructure, many sections of its non-proline-rich regions have cell or matrix clan/regulation roles. The relatively high content of proline and hydroxyproline rings, with their geometrically constrained carboxyl and (secondary) amino groups, along with the rich affluence of glycine, accounts for the tendency of the individual polypeptide strands to form left-handed helices spontaneously, without any intrachain hydrogen bonding.
Because glycine is the smallest amino acid with no side chain, it plays a unique role in fibrous structural proteins. In collagen, Gly is required at every tertiary position considering the assembly of the triple helix puts this residue at the interior (axis) of the helix, where there is no space for a larger side grouping than glycine's unmarried hydrogen atom. For the same reason, the rings of the Pro and Hyp must point outward. These two amino acids help stabilize the triple helix – Hyp fifty-fifty more than and so than Pro; a lower concentration of them is required in animals such every bit fish, whose body temperatures are lower than well-nigh warm-blooded animals. Lower proline and hydroxyproline contents are characteristic of cold-water, but non warm-water fish; the latter tend to have similar proline and hydroxyproline contents to mammals.[27] The lower proline and hydroxproline contents of common cold-water fish and other poikilotherm animals leads to their collagen having a lower thermal stability than mammalian collagen.[27] This lower thermal stability ways that gelatin derived from fish collagen is not suitable for many food and industrial applications.
The tropocollagen subunits spontaneously cocky-assemble, with regularly staggered ends, into fifty-fifty larger arrays in the extracellular spaces of tissues.[34] [35] Additional assembly of fibrils is guided by fibroblasts, which deposit fully formed fibrils from fibripositors. In the fibrillar collagens, molecules are staggered to adjacent molecules by near 67 nm (a unit that is referred to equally 'D' and changes depending upon the hydration state of the aggregate). In each D-menstruum repeat of the microfibril, in that location is a role containing v molecules in cross-section, called the "overlap", and a part containing merely four molecules, called the "gap".[36] These overlap and gap regions are retained as microfibrils gather into fibrils, and are thus viewable using electron microscopy. The triple helical tropocollagens in the microfibrils are arranged in a quasihexagonal packing design.[36] [37]
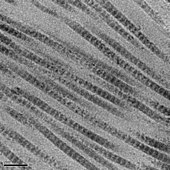
The D-menstruation of collagen fibrils results in visible 67nm bands when observed past electron microscopy.
There is some covalent crosslinking within the triple helices, and a variable amount of covalent crosslinking betwixt tropocollagen helices forming well organized aggregates (such equally fibrils).[38] Larger fibrillar bundles are formed with the aid of several dissimilar classes of proteins (including different collagen types), glycoproteins, and proteoglycans to form the different types of mature tissues from alternate combinations of the same key players.[35] Collagen'south insolubility was a bulwark to the report of monomeric collagen until it was found that tropocollagen from immature animals tin can be extracted considering information technology is not nevertheless fully crosslinked. However, advances in microscopy techniques (i.east. electron microscopy (EM) and atomic forcefulness microscopy (AFM)) and Ten-ray diffraction have enabled researchers to obtain increasingly detailed images of collagen structure in situ.[39] These subsequently advances are particularly important to better understanding the way in which collagen structure affects prison cell–cell and jail cell–matrix communication and how tissues are constructed in growth and repair and inverse in evolution and disease.[40] [41] For example, using AFM–based nanoindentation it has been shown that a single collagen fibril is a heterogeneous material along its axial management with significantly dissimilar mechanical properties in its gap and overlap regions, correlating with its different molecular organizations in these two regions.[42]
Collagen fibrils/aggregates are arranged in different combinations and concentrations in various tissues to provide varying tissue properties. In bone, unabridged collagen triple helices lie in a parallel, staggered array. 40 nm gaps betwixt the ends of the tropocollagen subunits (approximately equal to the gap region) probably serve as nucleation sites for the deposition of long, hard, fine crystals of the mineral component, which is hydroxylapatite (approximately) Ca10(OH)ii(PO4)6.[43] Type I collagen gives bone its tensile strength.
Associated disorders [edit]
Collagen-related diseases almost commonly arise from genetic defects or nutritional deficiencies that affect the biosynthesis, assembly, posttranslational modification, secretion, or other processes involved in normal collagen production.
| Type | Notes | Gene(due south) | Disorders |
| I | This is the virtually abundant collagen of the man body. It is present in Scar tissue, the cease product when tissue heals by repair. It is establish in tendons, pare, avenue walls, cornea, the endomysium surrounding muscle fibers, fibrocartilage, and the organic office of bones and teeth. | COL1A1, COL1A2 | Osteogenesis imperfecta, Ehlers–Danlos syndrome, infantile cortical hyperostosis a.k.a. Caffey's affliction |
| Ii | Hyaline cartilage, makes up 50% of all cartilage protein. Vitreous humour of the eye. | COL2A1 | Collagenopathy, types II and 11 |
| III | This is the collagen of granulation tissue and is produced apace by young fibroblasts earlier the tougher type I collagen is synthesized. Reticular fiber. Also plant in artery walls, skin, intestines and the uterus | COL3A1 | Ehlers–Danlos syndrome, Dupuytren'south contracture |
| Iv | Basal lamina; eye lens. Also serves as function of the filtration system in capillaries and the glomeruli of nephron in the kidney. | COL4A1, COL4A2, COL4A3, COL4A4, COL4A5, COL4A6 | Alport syndrome, Goodpasture'south syndrome |
| V | Most interstitial tissue, assoc. with type I, associated with placenta | COL5A1, COL5A2, COL5A3 | Ehlers–Danlos syndrome (classical) |
| 6 | Most interstitial tissue, assoc. with blazon I | COL6A1, COL6A2, COL6A3, COL6A5 | Ulrich myopathy, Bethlem myopathy, atopic dermatitis[44] |
| Vii | Forms anchoring fibrils in dermoepidermal junctions | COL7A1 | Epidermolysis bullosa dystrophica |
| 8 | Some endothelial cells | COL8A1, COL8A2 | Posterior polymorphous corneal dystrophy ii |
| IX | FACIT collagen, cartilage, assoc. with type Ii and XI fibrils | COL9A1, COL9A2, COL9A3 | EDM2 and EDM3 |
| X | Hypertrophic and mineralizing cartilage | COL10A1 | Schmid metaphyseal dysplasia |
| XI | Cartilage | COL11A1, COL11A2 | Collagenopathy, types Ii and XI |
| XII | FACIT collagen, interacts with blazon I containing fibrils, decorin and glycosaminoglycans | COL12A1 | – |
| Thirteen | Transmembrane collagen, interacts with integrin a1b1, fibronectin and components of basement membranes similar nidogen and perlecan. | COL13A1 | – |
| Fourteen | FACIT collagen, besides known as undulin | COL14A1 | – |
| XV | – | COL15A1 | – |
| XVI | – | COL16A1 | – |
| XVII | Transmembrane collagen, likewise known equally BP180, a 180 kDa protein | COL17A1 | Bullous pemphigoid and certain forms of junctional epidermolysis bullosa |
| XVIII | Source of endostatin | COL18A1 | – |
| 19 | FACIT collagen | COL19A1 | – |
| Xx | – | COL20A1 | – |
| XXI | FACIT collagen | COL21A1 | – |
| XXII | – | COL22A1 | – |
| XXIII | MACIT collagen | COL23A1 | – |
| XXIV | – | COL24A1 | – |
| XXV | – | COL25A1 | – |
| XXVI | – | EMID2 | – |
| XXVII | – | COL27A1 | – |
| XXVIII | – | COL28A1 | – |
| XXIX | Epidermal collagen | COL29A1 | Atopic dermatitis[45] |
In add-on to the above-mentioned disorders, excessive deposition of collagen occurs in scleroderma.
Diseases [edit]
Ane yard mutations have been identified in 12 out of more twenty types of collagen. These mutations can lead to various diseases at the tissue level.[46]
Osteogenesis imperfecta – Caused past a mutation in blazon i collagen, ascendant autosomal disorder, results in weak bones and irregular connective tissue, some cases tin can be mild while others can exist lethal. Mild cases have lowered levels of collagen type ane while astringent cases have structural defects in collagen.[47]
Chondrodysplasias – Skeletal disorder believed to be caused by a mutation in type 2 collagen, further enquiry is being conducted to ostend this.[48]
Ehlers-Danlos syndrome – Thirteen dissimilar types of this disorder, which lead to deformities in connective tissue, are known.[49] Some of the rarer types can be lethal, leading to the rupture of arteries. Each syndrome is caused by a different mutation. For example, the vascular type (vEDS) of this disorder is caused past a mutation in collagen type 3.[50]
Alport syndrome – Can be passed on genetically, usually as 10-linked dominant, merely also as both an autosomal dominant and autosomal recessive disorder, sufferers have problems with their kidneys and eyes, loss of hearing can as well develop during the childhood or boyish years.[51]
Knobloch syndrome – Caused past a mutation in the COL18A1 gene that codes for the production of collagen Eighteen. Patients nowadays with protrusion of the brain tissue and degeneration of the retina; an private who has family members suffering from the disorder is at an increased take a chance of developing it themselves since in that location is a hereditary link.[46]
Characteristics [edit]
Collagen is one of the long, fibrous structural proteins whose functions are quite unlike from those of globular proteins, such as enzymes. Tough bundles of collagen called collagen fibers are a major component of the extracellular matrix that supports near tissues and gives cells structure from the outside, simply collagen is also found inside certain cells. Collagen has nifty tensile force, and is the main component of fascia, cartilage, ligaments, tendons, bone and skin.[52] [53] Along with elastin and soft keratin, information technology is responsible for skin strength and elasticity, and its degradation leads to wrinkles that accompany aging.[12] It strengthens blood vessels and plays a role in tissue development. It is present in the cornea and lens of the eye in crystalline grade. It may be i of the most arable proteins in the fossil record, given that it appears to fossilize frequently, fifty-fifty in bones from the Mesozoic and Paleozoic.[54]
Uses [edit]

A salami and the collagen casing (below) it came in
Collagen has a wide multifariousness of applications, from food to medical.[55] For instance, it is used in corrective surgery and burn surgery. It is widely used in the form of collagen casings for sausages.[56] [57]
If collagen is bailiwick to sufficient denaturation, e.1000. by heating, the three tropocollagen strands separate partially or completely into globular domains, containing a unlike secondary structure to the normal collagen polyproline II (PPII), e.g. random coils. This process describes the germination of gelatin, which is used in many foods, including flavored gelatin desserts. As well nutrient, gelatin has been used in pharmaceutical, cosmetic, and photography industries. Information technology is also used as a dietary supplement.[58]
From the Greek for glue, kolla, the word collagen means "glue producer" and refers to the early process of boiling the skin and sinews of horses and other animals to obtain glue. Collagen adhesive was used by Egyptians about 4,000 years agone, and Native Americans used information technology in bows virtually 1,500 years ago. The oldest gum in the earth, carbon-dated as more than eight,000 years old, was found to be collagen – used as a protective lining on rope baskets and embroidered fabrics, to concur utensils together, and in crisscross decorations on human skulls.[59] Collagen usually converts to gelatin, but survived due to dry atmospheric condition. Animal glues are thermoplastic, softening once again upon reheating, then they are still used in making musical instruments such equally fine violins and guitars, which may have to be reopened for repairs – an awarding incompatible with tough, synthetic plastic adhesives, which are permanent. Fauna sinews and skins, including leather, take been used to make useful articles for millennia.
Gelatin-resorcinol-formaldehyde glue (and with formaldehyde replaced by less-toxic pentanedial and ethanedial) has been used to repair experimental incisions in rabbit lungs.[60]
History [edit]
The molecular and packing structures of collagen eluded scientists over decades of research. The get-go show that it possesses a regular construction at the molecular level was presented in the mid-1930s.[61] [62] Research then concentrated on the conformation of the collagen monomer, producing several competing models, although correctly dealing with the conformation of each individual peptide chain. The triple-helical "Madras" model, proposed by M. N. Ramachandran in 1955, provided an accurate model of fourth structure in collagen.[63] [64] [65] [66] [67] This model was supported by further studies of college resolution in the late 20th century.[68] [69] [lxx] [71]
The packing structure of collagen has non been defined to the same degree outside of the fibrillar collagen types, although it has been long known to exist hexagonal.[37] [72] [73] As with its monomeric structure, several conflicting models propose either that the packing arrangement of collagen molecules is 'canvas-like', or is microfibrillar.[74] [75] The microfibrillar structure of collagen fibrils in tendon, cornea and cartilage was imaged directly past electron microscopy in the late 20th century and early 21st century.[76] [77] [78] The microfibrillar construction of rat tail tendon was modeled equally beingness closest to the observed structure, although it oversimplified the topological progression of neighboring collagen molecules, and so did not predict the correct conformation of the discontinuous D-periodic pentameric arrangement termed microfibril.[36] [79] [80]
See also [edit]
- Collagen hybridizing peptide, a peptide that tin demark to denatured collagen
- Hypermobility spectrum disorder
- Metalloprotease inhibitor
- Osteoid, collagen-containing component of bone
References [edit]
- ^ Di Lullo, Gloria A.; Sweeney, Shawn Yard.; Körkkö, Jarmo; Ala-Kokko, Leena & San Antonio, James D. (2002). "Mapping the Ligand-bounden Sites and Disease-associated Mutations on the Nigh Arable Protein in the Human, Type I Collagen". J. Biol. Chem. 277 (half dozen): 4223–31. doi:x.1074/jbc.M110709200. PMID 11704682.
- ^ "Leather grown using biotechnology is about to hitting the catwalk". The Economist. 26 August 2017. Archived from the original on 1 September 2017. Retrieved two September 2017.
- ^ Britannica Concise Encyclopedia 2007
- ^ Sikorski, Zdzisław East. (2001). Chemical and Functional Properties of Food Proteins. Boca Raton, Florida: CRC Press. p. 242. ISBN978-one-56676-960-0.
- ^ Bogue, Robert H. (1923). "Conditions Affecting the Hydrolysis of Collagen to Gelatin". Industrial and Engineering Chemistry. fifteen (11): 1154–59. doi:x.1021/ie50167a018.
- ^ O.E.D. second Edition 2005
- ^ Müller, Werner East. Thou. (2003). "The Origin of Metazoan Complication: Porifera as Integrated Animals". Integrative and Comparative Biological science. 43 (i): 3–10. CiteSeerX10.1.one.333.3174. doi:x.1093/icb/43.one.3. PMID 21680404. S2CID 17232196.
- ^ Sabiston textbook of surgery board review, 7th edition. Chapter 5 wound healing, question 14
- ^ a b Ricard-Blum, S. (2011). "The Collagen Family". Common cold Leap Harbor Perspectives in Biology. 3 (1): a004978. doi:x.1101/cshperspect.a004978. PMC3003457. PMID 21421911.
- ^ Franzke, CW; Bruckner, P; Bruckner-Tuderman, L (11 February 2005). "Collagenous transmembrane proteins: recent insights into biology and pathology". The Journal of Biological Chemical science. 280 (half-dozen): 4005–08. doi:ten.1074/jbc.R400034200. PMID 15561712.
- ^ Ashokkumar, Meiyazhagan; Ajayan, Pulickel Thousand. (3 April 2021). "Materials science perspective of multifunctional materials derived from collagen". International Materials Reviews. 66 (3): 160–87. doi:10.1080/09506608.2020.1750807. ISSN 0950-6608. S2CID 216270520.
- ^ a b Dermal Fillers | The Ageing Skin Archived thirteen May 2011 at the Wayback Car. Pharmaxchange.info. Retrieved on 21 April 2013.
- ^ a b Tiwari, D. P. (September 2015). "Biomaterials: An Overview" (PDF). International Journal of Key and Applied Enquiry. 3 (half dozen): 19. Retrieved one June 2021.
- ^ Cunniffe, M; F O'Brien (2011). "Collagen scaffolds for orthopedic regenerative medicine". The Journal of the Minerals, Metals and Materials Order. 63 (4): 66–73. Bibcode:2011JOM....63d..66C. doi:ten.1007/s11837-011-0061-y. S2CID 136755815.
- ^ Geiger, Thousand (2003). "Collagen sponges for os regeneration with rhBMP-2". Avant-garde Drug Delivery Reviews. 55 (12): 1613–29. doi:10.1016/j.addr.2003.08.010. ISSN 0169-409X. PMID 14623404.
- ^ Bunyaratavej, Pintippa; Wang, Hom-Lay (2001). "Collagen Membranes: A Review". Journal of Periodontology. 72 (ii): 215–29. doi:10.1902/jop.2001.72.two.215. hdl:2027.42/141506. ISSN 0022-3492. PMID 11288796.
- ^ Drury, Jeanie L.; Mooney, David J. (2003). "Hydrogels for tissue engineering: scaffold pattern variables and applications". Biomaterials. 24 (24): 4337–51. doi:10.1016/S0142-9612(03)00340-5. ISSN 0142-9612. PMID 12922147.
- ^ Tonndorf, Robert; Aibibu, Dilbar; Cherif, Chokri (2020). "Collagen multifilament spinning". Materials Science and Engineering: C. 106: 110105. doi:ten.1016/j.msec.2019.110105. ISSN 0928-4931. PMID 31753356. S2CID 202227968.
- ^ Oliveira, S; R Ringshia; R Legeros; E Clark; L Terracio; C Teixeira Grand Yost (2009). "An improved collagen scaffold for skeletal regeneration". Journal of Biomedical Materials. 94 (2): 371–79. doi:10.1002/jbm.a.32694. PMC2891373. PMID 20186736.
- ^ Onkar, Singh; Gupta, Shilpi Singh; Soni, Mohan; Moses, Sonia; Shukla, Sumit; Mathur, Raj Kumar (Jan 2011). "Collagen Dressing Versus Conventional Dressings in Burn and Chronic Wounds: A Retrospective Study". Periodical of Cutaneous and Aesthetic Surgery. four (1): 12–16. doi:ten.4103/0974-2077.79180. PMC3081477. PMID 21572675.
- ^ Gould, L. J. (2016). "Topical Collagen-Based Biomaterials for Chronic Wounds: Rationale and Clinical Application". Advances in Wound Care. 5 (1): 19–31. doi:x.1089/wound.2014.0595. PMC4717516. PMID 26858912.
- ^ "Collagen and Rosehip Extract Sachet". Alaina Pharma. Archived from the original on 4 July 2016. Retrieved 31 May 2021.
- ^ Birbrair, Alexander; Zhang, Tan; Files, Daniel C.; Mannava, Sandeep; Smith, Thomas; Wang, Zhong-Min; Messi, Maria L.; Mintz, Akiva; Delbono, Osvaldo (6 November 2014). "Type-i pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner". Stem Cell Inquiry & Therapy. v (6): 122. doi:10.1186/scrt512. ISSN 1757-6512. PMC4445991. PMID 25376879.
- ^ Singh, O; SS Gupta; Grand Soni; S Moses; S Shukla; RK Mathur (2011). "Collagen dressing versus conventional dressings in burn and chronic wounds: a retrospective written report". Periodical of Cutaneous and Aesthetic Surgery. 4 (i): 12–sixteen. doi:10.4103/0974-2077.79180. PMC3081477. PMID 21572675.
- ^ Accident, Nathan (2009). "Cell culture: building a better matrix". Nature Methods. six (8): 619–22. doi:10.1038/nmeth0809-619. S2CID 33438539.
- ^ Brodsky, Barbara; Persikov, Anton 5. (1 January 2005). "Molecular Structure of the Collagen Triple Helix". Advances in Protein Chemistry. 70: 301–39. doi:10.1016/S0065-3233(05)70009-7. ISBN978-0120342709. ISSN 0065-3233. PMID 15837519.
- ^ a b c d e Szpak, Paul (2011). "Fish bone chemistry and ultrastructure: implications for taphonomy and stable isotope assay". Journal of Archaeological Science. 38 (12): 3358–72. doi:10.1016/j.jas.2011.07.022.
- ^ Peterkofsky, B (1991). "Ascorbate requirement for hydroxylation and secretion of procollagen: Relationship to inhibition of collagen synthesis in scurvy". American Periodical of Clinical Nutrition. 54 (6 Suppl): 1135S–40S. doi:10.1093/ajcn/54.half-dozen.1135s. PMID 1720597.
- ^ Gorres, Chiliad. L.; Raines, R. T. (2010). "Prolyl 4-hydroxylase". Crit. Rev. Biochem. Mol. Biol. 45 (2): 106–24. doi:10.3109/10409231003627991. PMC2841224. PMID 20199358.
- ^ Myllylä, R.; Majamaa, K.; Günzler, V.; Hanauske-Abel, H. K.; Kivirikko, K. I. (1984). "Ascorbate is consumed stoichiometrically in the uncoupled reactions catalyzed by propyl iv-hydroxylase and lysyl hydroxylase". J. Biol. Chem. 259 (nine): 5403–05. doi:10.1016/S0021-9258(18)91023-9. PMID 6325436.
- ^ Houck, J. C.; Sharma, V. G.; Patel, Y. M.; Gladner, J. A. (1968). "Consecration of Collagenolytic and Proteolytic Activities by AntiInflammatory Drugs in the Skin and Fibroblasts". Biochemical Pharmacology. 17 (10): 2081–xc. doi:10.1016/0006-2952(68)90182-2. PMID 4301453.
- ^ "preprocollagen". The Free Dictionary.
- ^ Al-Hadithy, H.; Isenberg, DA; et al. (1982). "Neutrophil office in systemic lupus erythematosus and other collagen diseases". Ann Rheum Dis. 41 (1): 33–38. doi:x.1136/ard.41.1.33. PMC1000860. PMID 7065727.
- ^ Hulmes, D. J. (2002). "Edifice collagen molecules, fibrils, and suprafibrillar structures". J Struct Biol. 137 (one–2): 2–10. doi:10.1006/jsbi.2002.4450. PMID 12064927.
- ^ a b Hulmes, D. J. (1992). "The collagen superfamily – diverse structures and assemblies". Essays Biochem. 27: 49–67. PMID 1425603.
- ^ a b c Orgel, J. P.; Irving, TC; et al. (2006). "Microfibrillar construction of type I collagen in situ". PNAS. 103 (24): 9001–05. Bibcode:2006PNAS..103.9001O. doi:10.1073/pnas.0502718103. PMC1473175. PMID 16751282.
- ^ a b Hulmes, D. J. & Miller, A. (1979). "Quasi-hexagonal molecular packing in collagen fibrils". Nature. 282 (5741): 878–80. Bibcode:1979Natur.282..878H. doi:10.1038/282878a0. PMID 514368. S2CID 4332269.
- ^ Perumal, S.; Antipova, O. & Orgel, J. P. (2008). "Collagen fibril compages, domain arrangement, and triple-helical conformation govern its proteolysis". PNAS. 105 (viii): 2824–29. Bibcode:2008PNAS..105.2824P. doi:10.1073/pnas.0710588105. PMC2268544. PMID 18287018.
- ^ Buchanan, Jenna K.; Zhang, Yi; Holmes, Geoff; Covington, Anthony D.; Prabakar, Sujay (2019). "Office of X-ray Scattering Techniques in Understanding the Collagen Structure of Leather" (PDF). ChemistrySelect. 4 (48): 14091–102. doi:10.1002/slct.201902908. ISSN 2365-6549. S2CID 212830367.
- ^ Sweeney, S. M.; Orgel, JP; et al. (2008). "Candidate Cell and Matrix Interaction Domains on the Collagen Fibril, the Predominant Protein of Vertebrates". J Biol Chem. 283 (30): 21187–97. doi:10.1074/jbc.M709319200. PMC2475701. PMID 18487200.
- ^ Twardowski, T.; Fertala, A.; et al. (2007). "Blazon I collagen and collagen mimetics as angiogenesis promoting superpolymers". Curr Pharm Des. xiii (35): 3608–21. doi:ten.2174/138161207782794176. PMID 18220798.
- ^ Minary-Jolandan, M; Yu, MF (2009). "Nanomechanical heterogeneity in the gap and overlap regions of type I collagen fibrils with implications for bone heterogeneity". Biomacromolecules. 10 (ix): 2565–70. doi:10.1021/bm900519v. PMID 19694448.
- ^ Ross, 1000. H. and Pawlina, W. (2011) Histology, 6th ed., Lippincott Williams & Wilkins, p. 218.
- ^ Söderhäll, C.; Marenholz, I.; Kerscher, T.; Rüschendorf, F; Rüschendorf, F.; Esparza-Gordillo, J.; Mayr, Chiliad; et al. (2007). "Variants in a Novel Epidermal Collagen Gene (COL29A1) Are Associated with Atopic Dermatitis". PLOS Biological science. five (9): e242. doi:10.1371/journal.pbio.0050242. PMC1971127. PMID 17850181.
- ^ "Collagen Types and Linked Disorders". News-Medical.net. 18 January 2011. Archived from the original on 1 Dec 2017. Retrieved 19 Nov 2017.
- ^ a b Mahajan VB, Olney AH, Garrett P, Chary A, Dragan E, Lerner M, Murray J, Bassuk AG (2010). "Collagen Eighteen mutation in Knobloch syndrome with acute lymphoblastic leukemia". American Journal of Medical Genetics Office A. 152A (11): 2875–79. doi:10.1002/ajmg.a.33621. PMC2965270. PMID 20799329.
- ^ Gajko-Galicka, A (2002). "Mutations in type I collagen genes resulting in osteogenesis imperfecta in humans" (PDF). Acta Biochimica Polonica. 49 (2): 433–41. doi:10.18388/abp.2002_3802. PMID 12362985. Archived (PDF) from the original on seven June 2013.
- ^ Horton WA, Campbell D, Machado MA, Chou J (1989). "Type Two collagen screening in the human chondrodysplasias". Am. J. Med. Genet. 34 (4): 579–83. doi:10.1002/ajmg.1320340425. PMID 2624272.
- ^ Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, Bloom Fifty, Bowen JM, Brady AF, Burrows NP, Castori Grand, Cohen H, Colombi 1000, Demirdas Due south, De Backer J, De Paepe A, Fournel-Gigleux Due south, Frank Yard, Ghali N, Giunta C, Grahame R, Hakim A, Jeunemaitre X, Johnson D, Juul-Kristensen B, Kapferer-Seebacher I, Kazkaz H, Kosho T, Lavallee ME, Levy H, Mendoza-Londono R, Pepin M, Pope FM, Reinstein E, Robert L, Rohrbach M, Sanders L, Sobey GJ, Van Damme T, Vandersteen A, van Mourik C, Voermans N, Wheeldon N, Zschocke J, Tinkle B. 2017. The 2017 international classification of the Ehlers–Danlos syndromes. Am J Med Genet Part C Semin Med Genet 175C:8–26.
- ^ Hamel BC, Pals G, Engels CH, van den Akker E, Boers GH, van Dongen PW, Steijlen PM (1998). "Ehlers-Danlos syndrome and type III collagen abnormalities: a variable clinical spectrum". Clin. Genet. 53 (half-dozen): 440–46. doi:10.1111/j.1399-0004.1998.tb02592.ten. PMID 9712532. S2CID 39089732.
- ^ Kashtan, CE (1993) "Collagen Iv-Related Nephropathies (Alport Syndrome and Thin Basement Membrane Nephropathy Archived 2017-12-25 at the Wayback Machine)", in RA Pagon, TD Bird, CR Dolan, K Stephens & MP Adam (eds), GeneReviews, University of Washington, Seattle, Seattle WA PMID 20301386.
- ^ Fratzl, P. (2008). Collagen: Construction and Mechanics. New York: Springer. ISBN978-0-387-73905-2.
- ^ Buehler, M. J. (2006). "Nature designs tough collagen: Explaining the nanostructure of collagen fibrils". PNAS. 103 (33): 12285–90. Bibcode:2006PNAS..10312285B. doi:10.1073/pnas.0603216103. PMC1567872. PMID 16895989.
- ^ Zylberberg, L.; Laurin, M. (2011). "Analysis of fossil bone organic matrix by transmission electron microscopy". Comptes Rendus Palevol. xi (5–half dozen): 357–66. doi:10.1016/j.crpv.2011.04.004.
- ^ Penn Medicine (4 Nov 2018). "iv Head-To-Toe Means That Collagen Tin can Improve Your Health". Penn Medicine. Philadelphia, PA: Trustees of the University of Pennsylvania. Retrieved 7 June 2021.
- ^ "Understanding Collagen Casings | LEM Products | the Leader in Game Processing".
- ^ "What are Collagen Casings? How Are Collagen Casings Produced?".
- ^ Haug, I. J. (2011). Handbook of Food Proteins. Woodhead Publishing Limited. pp. 92–115. ISBN978-1-84569-758-vii.
- ^ Walker, Amélie A. (21 May 1998). "Oldest Mucilage Discovered". Archaeology. Archived from the original on 17 Dec 2005.
- ^ Ennker, I. C.; Ennker, JüRgen; et al. (1994). "Formaldehyde-gratuitous collagen mucilage in experimental lung gluing". Ann. Thorac. Surg. 57 (half-dozen): 1622–27. doi:10.1016/0003-4975(94)90136-8. PMID 8010812. Archived from the original on viii July 2012.
- ^ Wyckoff, R.; Corey, R. & Biscoe, J. (1935). "Ten-ray reflections of long spacing from tendon". Science. 82 (2121): 175–76. Bibcode:1935Sci....82..175W. doi:10.1126/scientific discipline.82.2121.175. PMID 17810172.
- ^ Clark, G.; Parker, E.; Schaad, J. & Warren, West. J. (1935). "New measurements of previously unknown large interplanar spacings in natural materials". J. Am. Chem. Soc. 57 (8): 1509. doi:10.1021/ja01311a504.
- ^ Ramachandran, 1000. N.; Kartha, Gopinath (September 1955). "Structure of Collagen". Nature. 176 (4482): 593–595. Bibcode:1955Natur.176..593R. doi:10.1038/176593a0. PMID 13265783. S2CID 33745131.
- ^ Ramachandran, Thousand. N.; Kartha, G. (August 1954). "Structure of Collagen". Nature. 174 (4423): 269–270. Bibcode:1954Natur.174..269R. doi:10.1038/174269c0. PMID 13185286. S2CID 4284147.
- ^ Balasubramanian, D . (October 2001). "GNR – A Tribute". Resonance. 6 (x): ii–iv. doi:10.1007/BF02836961. S2CID 122261106. Archived from the original on 10 Jan 2014.
- ^ Leonidas, Demetres D.; Chavali, GB; et al. (2001). "Binding of phosphate and pyrophosphate ions at the active site of homo angiogenin as revealed by X-ray crystallography". Protein Science. 10 (8): 1669–76. doi:ten.1110/ps.13601. PMC2374093. PMID 11468363.
- ^ Subramanian, Easwara (2001). "Obituary: 1000.N. Ramachandran". Nature Structural & Molecular Biology. 8 (six): 489–91. doi:10.1038/88544. PMID 11373614. S2CID 7231304.
- ^ Fraser, R. D.; MacRae, T. P. & Suzuki, E. (1979). "Concatenation conformation in the collagen molecule". J Mol Biol. 129 (3): 463–81. doi:10.1016/0022-2836(79)90507-2. PMID 458854.
- ^ Okuyama, Chiliad.; Okuyama, K; et al. (1981). "Crystal and molecular structure of a collagen-like polypeptide (Pro-Pro-Gly)x". J Mol Biol. 152 (two): 427–43. doi:10.1016/0022-2836(81)90252-7. PMID 7328660.
- ^ Traub, Westward.; Yonath, A. & Segal, D. Yard. (1969). "On the molecular structure of collagen". Nature. 221 (5184): 914–17. Bibcode:1969Natur.221..914T. doi:10.1038/221914a0. PMID 5765503. S2CID 4145093.
- ^ Bella, J.; Eaton, Thousand.; Brodsky, B.; Berman, H. One thousand. (1994). "Crystal and molecular construction of a collagen-like peptide at one.9 A resolution". Science. 266 (5182): 75–81. Bibcode:1994Sci...266...75B. doi:10.1126/scientific discipline.7695699. PMID 7695699.
- ^ Jesior, J. C.; Miller, A. & Berthet-Colominas, C. (1980). "Crystalline three-dimensional packing is general feature of type I collagen fibrils". FEBS Lett. 113 (ii): 238–xl. doi:10.1016/0014-5793(80)80600-4. PMID 7389896. S2CID 40958154.
- ^ Fraser, R. D. B. & MacRae, T. P. (1981). "Unit jail cell and molecular connectivity in tendon collagen". Int. J. Biol. Macromol. iii (iii): 193–200. doi:10.1016/0141-8130(81)90063-five.
- ^ Fraser, R. D.; MacRae, T. P.; Miller, A. (1987). "Molecular packing in type I collagen fibrils". J Mol Biol. 193 (1): 115–25. doi:10.1016/0022-2836(87)90631-0. PMID 3586015.
- ^ Wess, T. J.; Hammersley, AP; et al. (1998). "Molecular packing of type I collagen in tendon". J Mol Biol. 275 (ii): 255–67. doi:10.1006/jmbi.1997.1449. PMID 9466908.
- ^ Raspanti, Yard.; Ottani, 5.; Ruggeri, A. (1990). "Subfibrillar compages and functional backdrop of collagen: a comparative study in rat tendons". J. Anat. 172: 157–64. PMC1257211. PMID 2272900.
- ^ Holmes, D. F.; Gilpin, C. J.; Baldock, C.; Ziese, U.; Koster, A. J.; Kadler, Grand. E. (2001). "Corneal collagen fibril structure in three dimensions: Structural insights into fibril assembly, mechanical properties, and tissue organization". PNAS. 98 (xiii): 7307–12. Bibcode:2001PNAS...98.7307H. doi:x.1073/pnas.111150598. PMC34664. PMID 11390960.
- ^ Holmes, D. F.; Kadler, KE (2006). "The ten+4 microfibril structure of thin cartilage fibrils". PNAS. 103 (46): 17249–54. Bibcode:2006PNAS..10317249H. doi:x.1073/pnas.0608417103. PMC1859918. PMID 17088555.
- ^ Okuyama, Chiliad; Bächinger, HP; Mizuno, Yard; Boudko, SP; Engel, J; Berisio, R; Vitagliano, 50 (2009). "Comment on Microfibrillar structure of type I collagen in situ past Orgel et al. (2006), Proc. Natl Acad. Sci. USA, 103, 9001–05". Acta Crystallographica Department D. 65 (Pt9): 1009–10. doi:10.1107/S0907444909023051. PMID 19690380.
- ^ Orgel, Joseph (2009). "On the packing structure of collagen: response 0to Okuyama et al.'s comment on Microfibrillar structure of type I collagen in situ". Acta Crystallographica Section D. D65 (9): 1009. doi:x.1107/S0907444909028741.
| | Wikimedia Commons has media related to Collagen. |
Source: https://en.wikipedia.org/wiki/Collagen
Posted by: fitzpatrickpegglind.blogspot.com


0 Response to "Where Is Collagen Found In The Skin"
Post a Comment